Tocotrienol-rich vitamin E from palm oil (Tocovid) and its effects in diabetes and diabetic retinopathy: a pilot phase II clinical trial
Abstract
Aim: To identify the effects of tocotrienol-rich vitamin E from palm oil (Tocovid) on diabetic retinopathy (DR) in patients with type 2 diabetes.
Materials and methods: The intervention group (n = 21) received 200 mg Tocovid twice daily while the control group (n = 22) received placebo twice daily for 8 weeks. Changes in retinal photography by conventional grading and novel quantification of retinal hemorrhage were assessed. Changes in serum biomarkers advanced glycation end products (AGE) general, sRAGE (soluble receptor of AGE), Nε-CML (specific type of AGE), and cystatin C were evaluated.
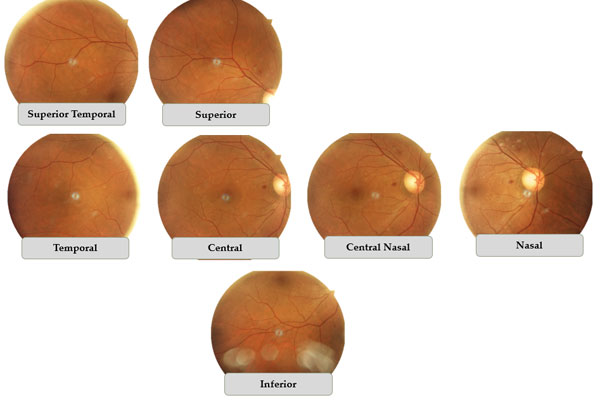
Results: A novel technique to quantify retinal hemorrhage had a strong positive correlation with conventional grading of DR in both eyes at baseline and at the end of the study. Eight-week supplementation of Tocovid resulted in significant reduction in retinal hemorrhage in the right eye. Liver enzymes and ALT significantly reduced. No significant changes in grade of DR, serum biomarkers, HbA1c, blood pressure, renal profile, and lipid profile were observed.
Conclusions: Tocovid is a potential adjunct to current treatment of DR and fatty liver disease. A novel method of quantifying retinal hemorrhage is a potential technique for assessing disease severity of DR, particularly the early changes.
References
Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564.
Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339-349.
Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290(15):2057-2060.
Montonen J, Knekt P, Järvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004 Feb;27(2):362-366.
Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther. 2011;36(1):53-63.
Xu R, Zhang S, Tao A, Chen G, Zhang M. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS One. 2014;9(4):e95008.
Peh HY, Tan WD, Liao W, Wong WF. Vitamin E therapy beyond cancer: Tocopherol versus tocotrienol. Pharmacol Ther. 2016;162:152-169.
Nazaimoon WW, Sakinah O, Gapor A, Khalid B. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr Res. 1996;16(11-12):1901-1911.
Nazaimoon WW, Khalid B. Tocotrienols-rich diet decreases advanced glycosylation endproducts in non-diabetic rats and improves glycemic control in streptozotocin-induced diabetic rats. Malays J Pathol. 2002;24(2):77-82.
Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of palm oil tocotrienol-rich fraction supplementation on biochemical parameters, oxidative stress and the vascular wall of streptozotocin-induced diabetic rats. Clinics. 2009;64(3):235-244.
Kanaya Y, Doi T, Sasaki H, et al. Rice bran extract prevents the elevation of plasma peroxylipid in KKAy diabetic mice. Diabetes Res Clin Prac. 2004;66: S157-S160.
Matough FA, Budin SB, Hamid ZA, Abdul-Rahman M, Al-Wahaibi N, Mohammed J. Tocotrienolrich fraction from palm oil prevents oxidative damage in diabetic rats. Sultan Qaboos Univ Med J. 2014;14(1): e95-103.
Pushparajah E, Rajadurai M. Effects of processing on the content and composition of tocopherols and tocotrienols in palm oil. International Conference on Palm Oil Product Technology in the Eighties; 1981 Jun 22-24; Special – Malaysian Agri (501), Kuala Lumpur: The Incorporated Society of Planters Malaysia; 1983.
Hashimoto T, Kato A, Tanabe K, et al. Studies on tocopherols and tocotrienols in Malaysian palm oil. Proceedings of International Symposium of the Advanced Industrial Utilisation of the Tropical Plants; 1980 Sep 1-4; Tsubuka, Japan: International Research and Development Cooperation Division, Ministry of International Trade and Industry; 1980.
Chida M, Suzuki K, Nakanishi-Ueda T, et al. In vitro testing of antioxidants and biochemical end-points in bovine retinal tissue. Ophthalmic Res. 1999;31(6):407-415.
Nakagawa K, Shibata A, Yamashita S, et al. In vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr. 2007;137(8):1938-43.
Cheng HS, Ton SH, Tan JB, Abdul Kadir KJ. The ameliorative effects of a tocotrienol-rich fraction on the AGE-RAGE axis and hypertension in high-fat-diet-fed rats with metabolic syndrome. Nutrients. 2017;9(9):984.
Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194-222.
Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
Genuth S, Sun W, Cleary P, et al. Glycation and carboxymethyllysine levels in skin collagen predict the risk of future 10-year progression of diabetic retinopathy and nephropathy in the diabetes control and complications trial and epidemiology of diabetes interventions and complications participants with type 1 diabetes. Diabetes. 2005;54(11):3103-3111.
Monnier VM, Bautista O, Kenny D, et al. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes. 1999;48(4):870-80.
The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;1993(329):977-986.
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
King P, Peacock I, Donnelly R. The UK Prospective Diabetes Study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br J Clin Pharmacol. 2001;48(5):643-648.
Choudhuri S, Dutta D, Sen A, et al. Role of N–epsilon-carboxy methyl lysine, advanced glycation end products and reactive oxygen species for the development of nonproliferative and proliferative retinopathy in type 2 diabetes mellitus. Mol Vis. 2013;19:100-113.
Boehm B, Schilling S, Rosinger S, et al. Elevated serum levels of Nε-carboxymethyl-lysine, an advanced glycation end product, are associated with proliferative diabetic retinopathy and macular oedema. Diabetologia. 2004;47(8):1376-1379.
Mishra N, Saxena S, Shukla RK, et al. Association of serum N ε-Carboxy methyl lysine with severity of diabetic retinopathy. J Diabetes Complications. 2016;30(3):511-517.
Kerkeni M, Saïdi A, Bouzidi H, Yahya SB, Hammami M. Elevated serum levels of AGEs, sRAGE, and pentosidine in Tunisian patients with severity of diabetic retinopathy. Microvasc Res. 2012;84(3):378-383.
Ng ZX, Chua KH, Iqbal T, Kuppusamy UR. Soluble receptor for advanced glycation end-product (sRAGE)/pentosidine ratio: a potential risk factor determinant for type 2 diabetic retinopathy. Int J Mol Sci. 2013;14(4):7480-7491.
Miranda ER, Fuller JK, Perkins R, et al. Soluble RAGE Sequesters Advanced Glycation End Products following an Overnight Fast in T1DM Patients. Diabetes. 2018;16(1).
Campion CG, Sanchez-Ferras O, Batchu SN. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Can J Kidney Health Dis. 2017;4:1-18.
Khatami PG, Soleimani A, Sharifi N, Aghadavod E, Asemi Z. The effects of high-dose vitamin E supplementation on biomarkers of kidney injury, inflammation, and oxidative stress in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. J Clin Lipidol. 2016;10(4):922-929.
Chen J, Ausayakhun S, Ausayakhun S, et al. Comparison of autophotomontage software programs in eyes with CMV retinitis. Invest Ophthalmol Vis Sci. 2011;52(13):9339-9344.
Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677-1682.
Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676-682.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193-203.
Jones S, Edwards RT. Diabetic retinopathy screening: a systematic review of the economic evidence. Diabet Med. 2010;27(3):249-256.
Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of diabetic retinopathy: XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112(9):1217-1228.
Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin epidemiologic study of diabetic retinopathy: XVII: The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801-1805.
Muto C, Yachi R, Aoki Y, Koike T, Igarashi O, Kiyose C. Gamma-tocotrienol reduces the triacylglycerol level in rat primary hepatocytes through regulation of fatty acid metabolism. J Clin Biochem Nutr. 2013;52(1):32-37.
Magosso E, Ansari MA, Gopalan Y, et al. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013;12(1):166.
Leong WH. Tocotrienol: the crown jewel in Malaysian red palm oil. Alternative Medicine. 2018;24:16-9
Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of nonâ€alcoholic fatty liver disease and nonâ€alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274-285.
Rosca MG, Mustata TG, Kinter MT, Ozdemir AM, Kern TS. Glycation of mitochondrial proteins from diabetic rat kidney is associated with excess superoxide formation. Am J Physiol Renal Physiol. 2005;289(2):F420-F430.
Nowotny K, Jung T, Höhn A, Weber D, Grune T. Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules. 2015;5(1):194-222.
The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560-2572.
The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129-139.
Ceriello A. The emerging challenge in diabetes: the “metabolic memoryâ€. Vasc Pharmacol. 2012;57(5-6):133-138.
Aschner PJ, Ruiz AJ. Metabolic memory for vascular disease in diabetes. Diabetes Technol Ther. 2012;14(S1):S68-S74.
Torok Z, Peto T, Csosz E, et al. Tear fluid proteomics multimarkers for diabetic retinopathy screening. BMC Ophthalmol. 2013;13(1):40.
Troughton RW, Frampton CM, Yandle TG, Espine EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355(9210):1126-1130.
Copyright (c) 2021 Yilynn Chiew, Suzanne May Quinn Tan, Badariah Ahmad, Sim Ee Khor, Khalid Abdul Kadir

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication, with the work twelve (12) months after publication simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).


